PH VALUES
The pH measures the acidity or alkalinity of water, with the value 7 representing
neutrality. Below this level the water is acid, and above it the water is alkaline
(or basic). Categorizing water as acid does not mean that it contains dangerous
acids. In forest streams and rivers the water accumulates with acid organic fluid
(humic acid) derived from the decomposition of plants (humus), producing an amber
yellow color.
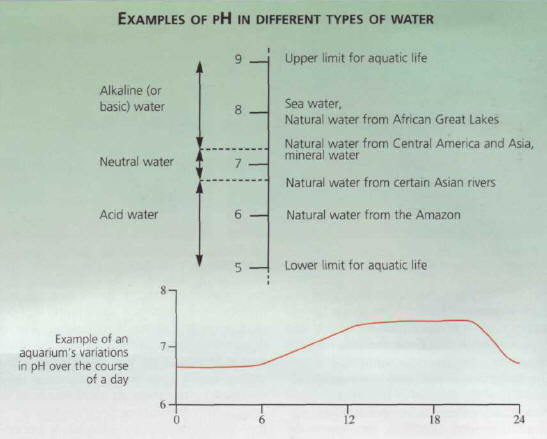
Generally speaking, aquatic life can exist only between pH 5 and 9. These extreme
values are rarely found in an aquarium, where the pH ranges from 6 to 8 according
to the type of water, and usually lies between 6.5 and 7.5. In aquariums, the term
acid water corresponds to a pH between 6 and 6.8, while alkaline water refers to
one between 7.2 and 8, and a pH between 6.8 and 7.2 is considered neutral. Variations
in pH are mainly the result of biological activity: the carbon dioxide produced
by living beings acidifies the water at night and the pH goes down slightly. Once
the carbon dioxide has been absorbed by the plants during the day the pH goes up
again.

pH is measured by using a color test: water from the aquarium
containing a few drops of the test is compared to a color scale that provides a
reasonably precise determination of the pH value. T
Although slight variations are therefore normal, more extreme changes can be
a warning signal. The pH is a good indicator of an aquarium's equilibrium, and it
should therefore be measured regularly. A colored marker dipped into a sample of
water is used to compare the color obtained with the scale provided. Electronic
meters are also now available for testing pH values.
Adjusting the pH
The pH of domestic water may not always be particularly suited to the fish you
have chosen. Furthermore, when an aquarium is in use the pH can rise and fall, slowly
but very regularly. There are some aquarium products on the market that enable adjustments
to be made to the pH, but there are other ways of modifying it.
- If the pH is too high
- the water can be diluted with another more acid water;
- the stirring of the water can be reduced. Carbon dioxide is eliminated
less quickly and remains in the water to acidify it. Be careful, because
decreasing the stirring also lowers the oxygenation;
- the water from the aquarium can be filtered over peat, which will release
certain acids. The amount of peat needed to maintain a specific pH value
must be found through trial and error, with regular measurements of the
pH.
- If the pH is too low
- the water can be diluted with another more alkaline, and generally harder
water (see Hardness, below);
- the agitation of the water can be increased, enhancing the elimination
of the carbon dioxide dissolved in the water and therefore lifting the pH;
- the water can be filtered over calcareous material, rock, or oyster
shells broken into little pieces. In this case, the hardness also increases
(see below).
|