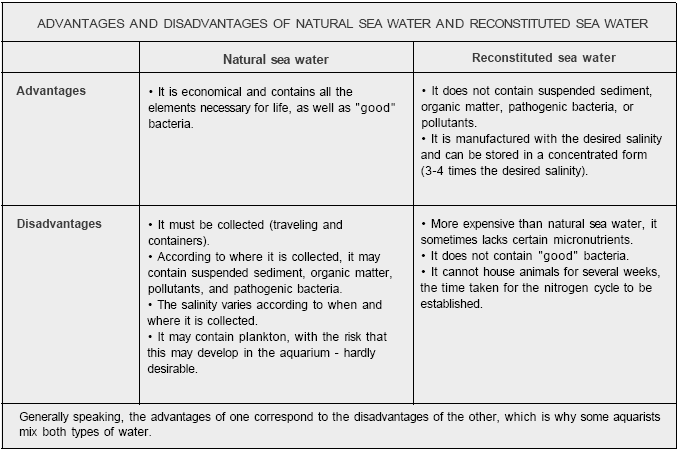
THE ORIGIN OF SALT WATER IN MARINE AQUARIUMS
The first idea which springs to mind is that of collecting natural sea water,
but this is difficult for somebody who lives a long way from a coast or requires
large quantities. Moreover, although sea water does present advantages, it also
has its inconveniences. While some aquarists filter it before using it to totally
or partially fill up their tanks, the majority use reconstituted sea water. In theory,
the recipe is a simple one: dissolve the salts in the water. In practice, however,
not just any water or any salts can be used, and it is out of the question to use
table salt or that derived from salt marshes. Furthermore, good sea water cannot
be reconstituted using poor quality fresh water.
Where and when to collect natural sea water?
The ideal solution would be to go to the open sea, where the water is likely
to be less polluted and to have more constant characteristics. Near the coasts,
the following must be avoided: urbanized or industrialized areas and ports, which
are susceptible to pollution; anywhere near river mouths, estuaries, or bays, where
the water is desalted; and areas of stagnant sea water (pools at low tide) and salt
marshes.
Coasts with sand dunes are suitable in principle, but the water is often laden
with suspended sediment. Rocky coasts are preferable regions from where water can
be collected.

The collection of water from a coastal area is to be avoided,
especially when the locality is used for dumping urban or industrial waste.
The best periods for collection are autumn and winter, because plankton develop
in spring and tourism increases the risk of pollution in summer. Calm weather is
preferable, in order to avoid suspended material, although a heavy swell reoxygenates
the water. In this case, the water can be collected 1-3 days later, the time in
which the suspended material turns into sediment. However, the water must be filtered
in all cases, first roughly and then more finely.

Salt collected in salt marshes is not suitable for reconstituting
sea water intended for an aquarium.
The reconstitution of artificial sea water
The quality of the fresh water used is important: it must be as pure as possible.
It is best to use water with a hardness of less than 8.4, although reconstitution
is still possible with higher levels, providing the CH is equal to at least 75-80%
of the general hardness value. Take care to avoid water containing nitrates (often
found in farming areas), to which invertebrates are very sensitive, or metals, toxic
for some animals where present above certain limits.
|